Getting this topic straight in your mind will make it much, much easier to grasp key concepts like why glucose dissolves in water, why other things don’t, and how neurons and mitrochondria use membranes to create ion gradients for their function. And you’ll need to understand hydrogen bonding of polar groups to understand how DNA and proteins adopt defined structures.
The fact the molecules are called ‘polar’ and ‘charged’ is part of the problem - this can be pretty confusing! So don’t rely on their names to understand what’s going on.
Let’s start from scratch:
How are Polar and Charged Molecules different from Non-Polar, Uncharged Molecules?
All molecules contain atoms. And all atoms contain positively-charged nuclei and negatively-charged electrons.
In a non-polar, non-charged molecule, these positive and negative charges all neatly cancel each other out. As far as other nearby molecules are concerned, a non-polar molecule behaves as though it has no charges at all.
In both polar and charged molecules, the molecule has regions of positive and/or negative charges that can affect nearby molecules (or even other parts of the same molecule - as happens in proteins and DNA).
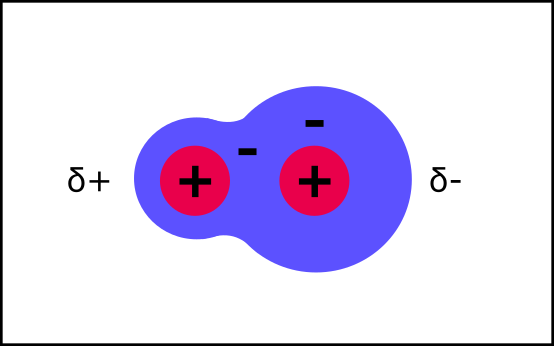
What’s the difference between Polar and Charged Molecules?
Polar molecules are charged-balanced overall but have unevenly distributed electrons. This gives them a little bit of a charge in certain places.
Charged molecules do have an overall charge. They have at leat one full unit of charge on at least one atom. (A unit of charge being equal to the magnitude of one electron).
You will also hear about polar and charged groups, which are a part of a larger molecule, where that part (group of atoms) has these properties.
Now, you might read that and think yes! I’ve got it! But to really understand it - and more importantly to remember it - you are going to need to linger a while and spend a bit of time thinking about this. It’s worth going through it all carefully step by step - this will also check your understanding. Have a good think about where those electrons are. Too many students trip up on this topic.
So let’s look at what it means to be non-polar, polar or charged. And then how that affects the behaviour of these molecules.
First step: What’s the difference between Unpolar and Polar Molecules