The similarites and differences between non-polar, polar and charged molecules (or parts of molecules) are really important. You must understand the difference between polar and charged molecules if you are going to make sense of molecular structure, and of the ways in which molecules interact.
If you’re unsure if water is a polar molecule or are wondering whether ions are polar, this article is for you.
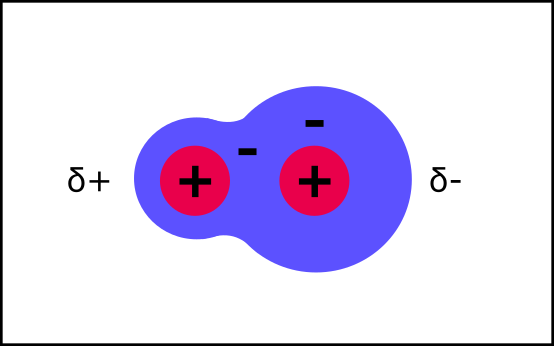
This molecule is polar, but not charged. All is explained below.
Getting this topic straight in your mind will make it much, much easier to grasp key concepts like why glucose dissolves in water, why other things don’t, and how neurons and mitrochondria use membranes to create ion gradients for their function. And you’ll need to understand hydrogen bonding of polar groups to understand how DNA and proteins adopt defined structures.
The fact the molecules are called ‘polar’ and ‘charged’ is part of the problem - this can be pretty confusing! So don’t rely on their names to understand what’s going on.
Let’s start from scratch:
How are Polar and Charged Molecules different from Non-Polar, Uncharged Molecules?
All molecules contain atoms. And all atoms contain positively-charged nuclei and negatively-charged electrons.
In a non-polar, non-charged molecule, these positive and negative charges all neatly cancel each other out. As far as other nearby molecules are concerned, a non-polar molecule behaves as though it has no charges at all.
In both polar and charged molecules, the molecule has regions of positive and/or negative charges that can affect nearby molecules (or even other parts of the same molecule - as happens in proteins and DNA).
What’s the difference between Polar and Charged Molecules?
Polar molecules are charged-balanced overall but have unevenly distributed electrons. This gives them a little bit of a charge in certain places.
Charged molecules do have an overall charge. They have at leat one full unit of charge on at least one atom. (A unit of charge being equal to the magnitude of one electron).
You will also hear about polar and charged groups, which are a part of a larger molecule, where that part (group of atoms) has these properties.
Now, you might read that and think yes! I’ve got it! But to really understand it - and more importantly to remember it - you are going to need to linger a while and spend a bit of time thinking about this. It’s worth going through it all carefully step by step - this will also check your understanding. Have a good think about where those electrons are. Too many students trip up on this topic.
So let’s look at what it means to be non-polar, polar or charged. And then how that affects the behaviour of these molecules.
First step: What’s the difference between Unpolar and Polar Molecules
Atom
The atoms that make up molecules each have a postively-charged nucleus and a cloud of negatively-charged electrons.
Different types of atoms have different numbers of charges.
This means that even non-charged, non-polar molecules contain charges! They just cancel each other out so you don’t notice them.
Non-polar
When you make a molecule out of atoms, electrons are shared between neighbouring atoms. The electrons become one big shared cloud. This makes a covalent bond.
The diagram shows a non-polar molecule with two atoms. In a non-polar molecule, all the charges are balanced, cancelling each other out.
Because the charges are distributed evenly, and cancel out overall, the molecule behaves as though there are no charges (in terms of its electrostatic interactions with other nearby molecules).
So why are they called “non-polar”? To understand that, you need to understand what polar means.
Polar
It turns out that some types of atomic nuclei just LOVE electrons. Like, they are particularly greedy for them. Oxygen, for example.
These greedy atoms yank the electron cloud over towards their nucleus, away from the nucleus of the other atom.
The other atom no longer has enough negative charge to cancel out its positively-charged nucleus. While the greedy one has more negatively charged electrons than it needs.
The charges no longer cancel each other out. The other atom now has just a little bit of a positive charge, and the greedy one has just a little bit of a negative charge.
This is a polar molecule. Its atoms still share one electron cloud, so they are still covalently bonded. But the small charge in charge distribution mean it will now interact differently with its environment.
Overall, the charges still cancel out. They are just unbalanced so that there are places with just a little bit of charge.
Saying "just a little bit” of charge is a pain, so instead the delta symbol is used to show this.
δ+ = just a little bit of positive charge
δ- = just a little bit of negative charge
This can also happen to just one part of a molecule. A good example is a hydroxyl group (OH). The oxygen pulls the electrons toward it, so that there is just a little bit (δ) of charge on the oxygen and hydrogen atoms.
Molecules with hydroxyl groups are polar. Look at glucose - it has loads of hydroxyl groups; this is what makes it a polar molecule. This is important for how it behaves in water, but before we get to that, let’s look at how charged atoms/molecules are different:
Second step: What’s the Difference between Polar and Charged Molecules?
Polar
A polar molecule has no overall charge. The charge of its positive nuclei exactly cancel out the charge of its negative electrons.
The charges are just unevenly distributed, giving a little bit (δ) of positive charge to one atom, and slight negative charge to another atom.
In biology, you’ll normally find it’s a hydrogen atom that has had its electrons yanked away and is now carrying a little bit of positive charge.
Charged
Now look at this. These atoms are not sharing a cloud of electrons - the big one has gone all-in and taken the whole lot for itself.
No shared electron cloud means there is no covalent bond.
No covalent bond means they are no longer a single molecule, but rather two separate atoms … well, except that they’re not even atoms any more …
The atoms no longer just have just a little bit (δ) of charge. The one on the left has lost an entire electron’s worth of charge. Losing negative charge means that overall it is now (properly, not just a little bit) positively charged.
The one on the right has a whole electron’s worth of negative charge more than it needs to cancel out its positive nuclues. It is now (properly) negatively charged.
Because they are (properly!) charged, we no longer call them atoms. Instead they are ions.
Water experiences this sort of electron-theft.
Sometimes it exists as the polar H20 molecule, but sometimes its oxygen gets even more greedy and the molecule dissociates into H+ (a hydrogen ion, aka proton), and OH- (a hydroxyl ion).
This dissociation, and the reforming of H20, is happening all the time in normal liquid water.
Note that the ions each have an overall charge, unlike the polar water molecule where the small charges cancel out.
Bigger Molecules
Atoms that are negatively charged due to having extra electrons, or that are positively charged because they lack electrons, often occur in large molecules too.
Where positive charges are found, it helps to think about this as a positively-charged H+ having been added to the molcule.
Here’s an amine group. It’s just part of a larger molecule, which goes off the edge of the image.
It can exist either as —NH2, or it can add on a proton (H+) to become —NH3+.
In living organisms, there are plenty of available protons (remember how water dissociates?). So amine groups like this usually exist as the charged version.
This is not a polar group, it is charged. (Ignore the shape of the electron cloud for this one, the important thing is that there is an overall charge of +1 because of that extra proton).
Electrostatic Interactions
So. Polar molecules are uncharged overall but have just a little bit (δ) of charge in various places. While charged molecules have a big whack of charge due to having lost an electron or having gained a proton. Why is this difference so important?
It’s to do with how polar and charged molecules interact with their environments. It’s not the same.
Hydrogen bonds
Some polar molecules, like DNA, proteins and water, can form hydrogen bonds between the atoms that have the unevenly distributed charges. These are a special type of weak bond.
Water LOVES making hydrogen bonds - this is why it can hold itself together into a droplet.
Notice in the picture that the water molecules remain separate and can still move around. It doesn’t take much to pull a single hydrogen bond apart. Which is why water can still be poured and stirred around with no trouble.
Water is a polar, hydrogen-bonding molecule, and this explains its properties as a solvent. Molecules like glucose can dissolve in water because they are similarly polar and able to make hydrogen bonds.
Hydrogen bonds are also really important in understanding DNA and protein structures.
These molecules hydrogen-bond to themselves. Each individual bond is weak, but multiple repeating bonds work together to hold the structure into shape.
Protein secondary structures are held together by hydrogen bonds.
The image here shows hydrogen bonds between Guanine (G) and Cytosine (C) in DNA. The hydrogen bonds are shown as dotted lines.
To get a feeling for the strength of hydrogen bonds, think about what happens if you spill water on a book, close it, and let it dry. You know how the pages stick together? This is because hydrogen bonds have formed between the pressed-together pages. When you peel them apart, you are pulling these hydrogen bonds apart.
Dissolving ions
Charged ions like Cl-, Na+ and K+ can’t form hydrogen bonds, but they can still dissolve in water because they can form favourable electrostatic interactions with the water molecules.
This diagram shows salt (NaCl) dissolved in water.
Hydrogen bonds are in yellow. And electrostatic interactions between the charged ions and the polar water molecules are shown in green.
See how the water molecules organise around the ions to provide the opposite charge to that presented by the ion.
Non-polar molecules like lipids cannot form electrostatic interactions with water molecules. And so for this reason, non-polar molecules do not dissolve in water. If you could somehow spread a bunch of non-polar molecule through a glass of water, this would cause all sorts of problems because the water molecules next to the non-polar molecules would be unable to satisfy their charges. Water prefers to hydrogen bond to itself, and it would do so, squeezing the the non-polar molecules out to cluster together in undissolved lumps.
Whisk up a teaspoon of oil in a glass of water and watch - you can see this happening. The oil ends up as a separate layer on the surface. Or get a small glass of oil and carefully put a drop of water on top; the water will ball itself up, hydrogen-bonding to itself and minimising the amount of contact it needs to make with the oil.
This is why membranes don’t dissolve in the cytoplasm. The water molecules would much rather hang out with other water molecules where they can make all those lovely hydrogen bonds. Non-polar molecules are called hydrophobic, or “water-hating”, but to be honest that’s a bit unfair because really it’s the water is excluding them, rather than the other way around.
This also means that non-polar molecules can’t act as solvents for polar molecules or charged ions. The reason being the same: they can’t offer any way to satisfy the polar/charged molecules’ hankering for favourable electrostatic interactions. This is why ions (Na+, K+, H+ etc) cannot dissolve into, and move through, membranes. Which is absolutely vital to understand if you want to make sense of how neurons, mitochondria, and chloroplasts function (and many other things in biology besides).
Ionic bonds
Charged molecules have ‘proper’ charges. They interact more strongly through electrostatic interactions to form ionic bonds.
Here is a positively charged amine group (NH3+) forming an ionic bond with a negatively charged hydroxyl group (OH-).
They are not sharing an electron cloud, so this is not a covalent bond.
Maybe these charged groups are both parts of the same protein (ie from different R groups). If so, this interaction may be important in defining the protein’s tertiary structure.
Or maybe it’s an interaction between an enzyme and its substrate?
Ionic bonds are really important for controlling what binds with what - and what doesn’t. Negatively charged groups will repel other negative charges. And positive will repel positive. This prevents incorrect structures forming.
In summary:
Polar molecules are charge-balanced overall but have unevenly distributed electrons. This gives them a little bit ( δ ) of a negative charge on one atom, and a little bit ( δ ) of positive charge on another. In biology, these weak charges often form hydrogen bonds, or favourable electrostatic interactions with ions.
Charged molecules have an overall charge. They have at leat one full unit of charge on at least one atom. (A unit of charge being equal to the magnitude of one electron). These stronger charges can form ionic bonds with each other.
This article was written by Dr Jenny Shipway
If this post has been helpful, please like ❤️ below and share with your friends.