Mastering Carbohydrates: Your Complete Guide to OCR A Level Biology Specification 2.1.2 (d-g)
Essential Prior Knowledge to Recap
Before diving into these commonly tested topics, ensure you're confident with:
• Basic carbohydrate classification – understanding the terms monosaccharide, disaccharide and polysaccharide, and being able to distinguish between them
• Condensation and hydrolysis reactions – knowing that condensation joins molecules together by removing water, whilst hydrolysis breaks bonds by adding water
• The concept of monomers and polymers – recognising that large biological molecules are built from smaller repeating units
• Chemical bonding basics – understanding covalent bonds and how atoms share electrons to form stable molecules
• The relationship between structure and function – appreciating that the shape and properties of molecules determine their biological roles
Links to GCSE Content
These A Level topics build directly upon your GCSE foundation:
• GCSE carbohydrates – you learned that carbohydrates are made of carbon, hydrogen and oxygen; now you'll explore their precise molecular structures and glycosidic bonds •
GCSE enzymes and digestion – you studied how enzymes break down starch into sugars; now you'll understand the specific bonds being broken and formed
• GCSE cell structure – you know that plant cell walls provide strength; now you'll discover exactly how cellulose molecules create this rigidity through hydrogen bonding
This guide will take you through each specification point systematically, with two carefully selected examination questions for each. By working through these examples with their detailed mark schemes, you'll develop the precise knowledge and examination technique needed to excel in this topic.
Specification Point (d): The ring structure and properties of glucose as an example of a hexose monosaccharide and the structure of ribose as an example of a pentose monosaccharide
This specification point requires you to understand the detailed molecular structures of monosaccharides, distinguish between hexose and pentose sugars, and recognise the difference between α and β glucose.
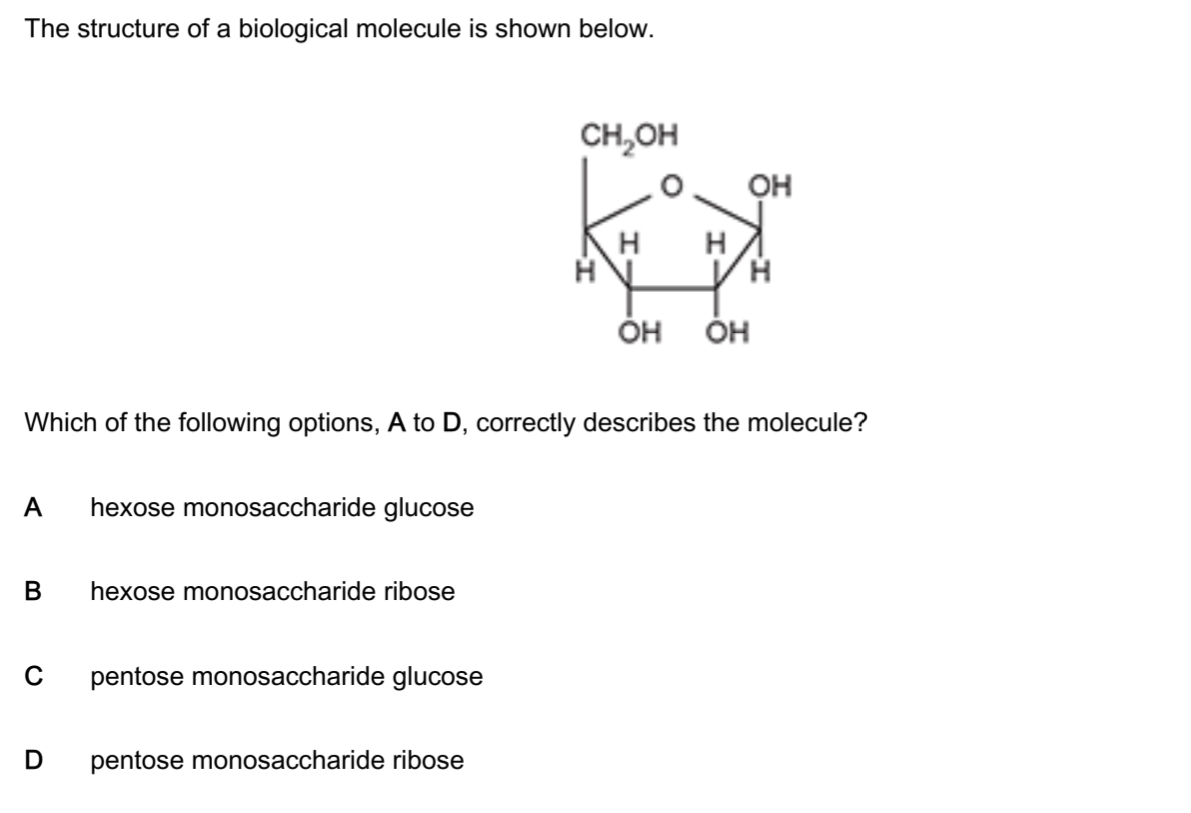
Example Question 1: Identifying a Pentose Monosaccharide
D – pentose monosaccharide ribose ✓
Detailed Explanation:
This question tests two crucial pieces of knowledge:
Can you count carbon atoms to distinguish pentose from hexose?
Can you name the common pentose and hexose monosaccharides?
Step 1: Count the Carbon Atoms
Looking at the structure carefully:
The ring contains 4 carbon atoms (shown at the corners of the ring where no other atom is labelled)
Plus 1 oxygen atom in the ring (the O shown in the ring)
Plus 1 carbon atom outside the ring as the CH₂OH group
Total = 5 carbon atoms
This is the critical observation: 5 carbons = pentose
Key Definitions:
Pentose = monosaccharide with 5 carbon atoms (penta = five)
General formula: C₅H₁₀O₅
Examples: ribose, deoxyribose, ribulose
Hexose = monosaccharide with 6 carbon atoms (hexa = six)
General formula: C₆H₁₂O₆
Examples: glucose, fructose, galactose
Step 2: Identify the Specific Pentose
Since we've established this is a pentose (5 carbons), we need to identify which pentose.
The most common pentose you need to know for A Level is ribose:
Ribose is found in RNA (ribonucleic acid)
Ribose is found in ATP (adenosine triphosphate)
Ribose forms a 5-membered ring (4 carbons + 1 oxygen)
Why Each Option is Right or Wrong:
Option A: "hexose monosaccharide glucose" ❌
Incorrect because: This molecule has 5 carbons, not 6
Glucose is indeed a hexose, but this structure isn't glucose
Double error: wrong number of carbons AND wrong name
Option B: "hexose monosaccharide ribose" ❌
Incorrect because: Ribose is NOT a hexose
Ribose always has 5 carbons (pentose)
This contradicts the basic definition of ribose
The structure shown does have 5 carbons, but calling it a hexose is wrong
Option C: "pentose monosaccharide glucose" ❌
Incorrect because: Glucose is NOT a pentose
Glucose always has 6 carbons (hexose)
The structure shown is a pentose, but glucose can never be a pentose
Contradicts the fundamental structure of glucose
Option D: "pentose monosaccharide ribose" ✓ CORRECT
Correct because:
The structure has 5 carbons → pentose ✓
Ribose is indeed a pentose ✓
Ribose forms this type of ring structure ✓
Everything matches perfectly
Understanding the Structural Differences:
Ribose (Pentose):
Contains 5 carbon atoms total
Ring formed from 4 carbons + 1 oxygen
1 carbon outside ring as CH₂OH
Formula: C₅H₁₀O₅
Glucose (Hexose):
Contains 6 carbon atoms total
Ring formed from 5 carbons + 1 oxygen
1 carbon outside ring as CH₂OH
Formula: C₆H₁₂O₆
The key difference is that glucose has one extra carbon in the ring compared to ribose.
How to Count Carbons in Ring Structures:
When you see a ring structure in organic chemistry:
Every "corner" or "vertex" without a letter is a carbon atom
If you see just bonds meeting at an angle, that's a carbon
In the structure shown, count the corners: 4 in the ring = 4 carbons
Count any carbon-containing groups outside the ring
CH₂OH = 1 carbon
CH₃ = 1 carbon
COOH = 1 carbon
Don't count oxygen, nitrogen, or other atoms as carbons!
The O in the ring is oxygen, not carbon
OH groups add 1 oxygen, not carbon
Add them all up
In this case: 4 (in ring) + 1 (CH₂OH) = 5 carbons total
Why This Distinction Matters in Biology:
Pentoses (like ribose):
Form the sugar-phosphate backbone of RNA and DNA
RNA contains ribose
DNA contains deoxyribose (ribose minus one oxygen)
Component of ATP (adenosine triphosphate) – the energy currency
Component of NADP and NAD – important coenzymes
Smaller size allows them to fit in nucleic acid structures
Hexoses (like glucose):
Primary respiratory substrates – broken down to release energy
Transported in blood and phloem sap
Polymerised to form storage polysaccharides (starch, glycogen)
Polymerised to form structural polysaccharides (cellulose)
Larger size stores more energy per molecule
Common Student Errors:
❌ Counting the oxygen in the ring as a carbon – this would give you 6 atoms in the ring, leading to confusion
❌ Not counting the CH₂OH carbon – remember this is a carbon atom outside the ring
❌ Confusing ribose with glucose – they're completely different molecules
❌ Thinking ribose can be a hexose – by definition, ribose is always C₅H₁₀O₅
❌ Thinking glucose can be a pentose – by definition, glucose is always C₆H₁₂O₆
Examiner's Comment from Mark Scheme:
"The correct response was D, however, all the other options were selected by different candidates."
This tells us that this question discriminates between candidates who:
Properly understand the definitions of pentose and hexose
Can correctly count carbon atoms in ring structures
Know the names of common monosaccharides
Memory Aids:
For remembering pentose = 5:
PENTose = PENTagon = 5 sides
PENTose = 5 carbons (both start with same sound)
For remembering hexose = 6:
HEXose = HEXagon = 6 sides
HEXose = 6 carbons
For specific molecules:
RIBOSE in RNA (both start with R)
GLUCOSE = GLUCose has 6 carbons (the word looks longer!)
Specification Learning Point:
This question directly addresses the specification requirement to know:
"the ring structure and properties of glucose as an example of a hexose monosaccharide"
"the structure of ribose as an example of a pentose monosaccharide"
You must be able to:
Recognise pentose vs hexose by counting carbons
Name ribose as the key pentose example
Name glucose as the key hexose example
Never confuse these categories – ribose is ALWAYS pentose, glucose is ALWAYS hexose
Practice Tip:
Draw both ribose and glucose structures side by side. Label them clearly:
Ribose: 5C (pentose)
Glucose: 6C (hexose)
Do this repeatedly until you can instantly distinguish them. This is tested frequently in multiple choice questions and is easy marks if you know it!
Example Question 2: Drawing the Structure of Alpha Glucose
(i) Write on the diagram to show the complete structure of alpha glucose. [3 marks]
Mark Scheme:
Correct positions for CH₂OH ✓ (1 mark)
O (oxygen) correctly positioned ✓ (1 mark)
OH and H groups correct on C1 ✓ (1 mark)
Guidance:
Allow bond line to any part of the group (doesn't need perfect attachment)
Allow correct displayed formula (showing all atoms and bonds)
Ignore bond angles (you won't lose marks for imperfect angles)
Model Answer:
The completed structure should show:
CH₂OH group attached to carbon-5, projecting upwards from the ring
Oxygen atom (O) in the ring between carbon-5 and carbon-1
H above and OH below on carbon-1 (this is the α configuration)
Complete Structure Explanation:
Let me walk you through building the complete α-glucose molecule systematically:
Step 1: The Ring Structure
The ring consists of 5 carbons and 1 oxygen
The oxygen sits between carbon-5 and carbon-1
The ring is not perfectly flat – it adopts a "chair" conformation
Step 2: Number the Carbons Working clockwise from the oxygen:
Carbon-1: The anomeric carbon (on the right, next to oxygen)
Carbon-2: Next position clockwise
Carbon-3: Next position clockwise
Carbon-4: Next position clockwise (at the bottom)
Carbon-5: Next to oxygen on the left
Carbon-6: Not in the ring – it's the CH₂OH group attached to C5
Step 3: Position Groups on Each Carbon
Carbon Group Above Group Below C1 H OH (defines α) C2 OH H C3 H OH C4 OH H C5 CH₂OH Part of ring
Step 4: The Critical α Feature On carbon-1, you must have:
H above the plane
OH below the plane
If these were reversed (OH above, H below), you'd have β-glucose instead.
Understanding the Three Marking Points:
Marking Point 1: CH₂OH Group (1 mark)
The CH₂OH group must be positioned correctly:
Attached to carbon-5 (the carbon to the left, next to the oxygen in the ring)
Projects upwards from the ring (in standard Haworth projection)
This is the 6th carbon of glucose (carbon-6)
Why this group matters:
This is what makes glucose a hexose – this is the 6th carbon
This group is involved in forming 1,6 glycosidic bonds in branched polysaccharides
It's a primary alcohol group (-CH₂OH rather than -CHOH)
Common errors:
❌ Putting CH₂OH on the wrong carbon
❌ Writing just CH₃ instead of CH₂OH
❌ Forgetting it entirely
Marking Point 2: Oxygen in the Ring (1 mark)
The oxygen atom must be:
Inside the ring (not outside)
Positioned between carbon-5 and carbon-1
Forms part of the ring structure itself
Why this matters:
Glucose is a cyclic hemiacetal – the ring forms when the -CHO group reacts with the -OH on C5
The oxygen in the ring comes from the -OH group originally on C5
This creates the ring form (which is the predominant form in aqueous solution)
Common errors:
❌ Leaving the oxygen out entirely (making it just a carbon ring)
❌ Putting oxygen outside the ring as OH groups
❌ Putting oxygen in the wrong position in the ring
Marking Point 3: OH and H on Carbon-1 (1 mark)
This is THE critical feature that defines α-glucose:
On carbon-1 (the anomeric carbon):
H must be above the plane of the ring
OH must be below the plane of the ring
Why this is crucial: This single difference distinguishes α from β:
Type Position on C1 Forms which polymers α-glucose OH below Starch, glycogen β-glucose OH above Cellulose
The Biological Consequence:
This seemingly tiny difference has ENORMOUS consequences:
α-glucose:
Forms α-glycosidic bonds in polymers
Creates starch (plants) and glycogen (animals)
We have enzymes (amylase, maltase) that can break these bonds
We can digest starch – that's why we can eat bread, pasta, potatoes, rice
β-glucose:
Forms β-glycosidic bonds in polymers
Creates cellulose (plant cell walls)
We don't have enzymes (cellulase) to break these bonds
We cannot digest cellulose – that's why we can't digest wood, grass, or paper
Common mistakes:
❌ Putting OH above on C1 – this creates β-glucose, not α-glucose (0 marks for this point)
❌ Putting both H and OH on the same side – chemically impossible
❌ Leaving C1 incomplete – you must show what's attached
❌ Forgetting which is which – use a memory aid!
Memory Aids for α vs β:
Method 1: Alphabet order
α (alpha) comes before β (beta) in the alphabet
α has OH below (down = comes before)
β has OH above (up = comes after)
Method 2: Visual
α = Away (OH points away, down from the CH₂OH group)
β = Both up (Both OH and CH₂OH point up, same side)
Method 3: Rhyme
"α is below, β makes it grow (up)"
How to Approach This Question in an Exam:
Step-by-step process:
First, add the oxygen in the ring (between C5 and C1)
This shows you understand it's a ring structure with oxygen
Next, add the CH₂OH to carbon-5 (projecting upwards)
This completes the hexose structure (6 carbons total)
Finally, complete carbon-1 with H above and OH below
Double-check this is α not β
This is the defining feature of α-glucose
Check all other carbons have their groups
C2: OH above, H below
C3: H above, OH below
C4: OH above, H below
(These may already be shown in the incomplete structure)
What the Question Doesn't Penalise:
According to the mark scheme, you won't lose marks for:
Imperfect bond angles (as long as connectivity is clear)
Slightly wonky ring shape
Bonds not perfectly straight
Groups not perfectly positioned as long as it's clear whether they're above or below
What WILL Lose Marks:
✗ Wrong position of CH₂OH (not on C5) ✗ Missing oxygen from the ring ✗ Wrong configuration on C1 (making it β-glucose) ✗ Missing groups entirely
Examiner's Insight:
"This question differentiated well between candidates. Around two-thirds got either the 'O' or the groups on 'C₁' correct and many candidates got both correct. A smaller proportion got the C₆ group correct but almost half achieved full marks. Some candidates, usually those who didn't perform well on the rest of the paper, achieved 0 marks."
What this tells us:
The question is doable – half of candidates got full marks
But it requires precise knowledge – you must know ALL three features
Candidates who didn't know glucose structure at all scored 0
This is a core skill you MUST master
Practice Strategy:
Draw α-glucose 10 times from memory – time yourself
Draw β-glucose 10 times from memory – compare to α
Draw them side by side and label the differences clearly
Cover them up and test yourself – can you draw both perfectly?
Use past paper questions – practice completing partial structures
Key Features of Complete α-Glucose:
6 carbons total (5 in ring + 1 as CH₂OH) = hexose
1 oxygen in the ring (between C5 and C1)
OH below on C1 = α-glucose (key defining feature)
CH₂OH on C5 projecting upwards
Specification Learning Point:
This question directly tests the specification requirement:
"the ring structure and properties of glucose as an example of a hexose monosaccharide"
You must be able to:
✓ Draw the complete ring structure
✓ Show it has 6 carbons (hexose)
✓ Distinguish α from β based on C1 configuration
✓ Complete partial structures accurately
This skill appears in multiple question types and is worth 3 marks – excellent return on investment if you learn it properly!
Specification Point (e): The synthesis and breakdown of a disaccharide and polysaccharide by the formation and breakage of glycosidic bonds
This specification point requires you to understand condensation and hydrolysis reactions, name glycosidic bonds precisely, and recognise specific disaccharides.
Example Question 1: Describing the Glycosidic Bond
Mark Scheme:
(α-)glycosidic (bond) ✓
carbon 1 to carbon 4 (bond) ✓
Alternative acceptable answers:
"(α-)1,4 glycosidic bond" gains both marks
"1,4 bond" gains mark 2 only
Guidance: ✓ Accept marks clearly shown on annotated diagram ✗ Do NOT allow "beta/β" ✗ Do NOT allow "1,6 bond" ✓ Allow "1,4 bond" for second mark ✗ ECF: "β-1,4 glycosidic bond" can get mark 2; "β-1,6 bond" = 0 marks ✗ Ignore references to any named carbohydrate
Model Answer:
"The bond is an α-glycosidic bond formed between carbon 1 of one glucose molecule and carbon 4 of the other glucose molecule."
Or more concisely:
"α-1,4 glycosidic bond"
Detailed Explanation:
Part 1: Bond Type (1 mark)
The bond type is glycosidic. This term is absolutely essential. Let's be clear about what this means:
A glycosidic bond is a covalent bond formed between two monosaccharides
It forms through a condensation reaction (removing H₂O)
The bond links through oxygen: C-O-C
It can be broken by hydrolysis (adding H₂O)
You must use the word "glycosidic" – simply saying "covalent bond" won't gain the mark, even though it's technically correct. The mark scheme requires the specific term.
The α (alpha) prefix indicates that the bond involves α-glucose monomers. Since maltose is made from two α-glucose molecules, it's an α-glycosidic bond.
Part 2: Carbon Positions (1 mark)
The bond forms between:
Carbon-1 of the first glucose (the anomeric carbon where the OH group is below in α-glucose)
Carbon-4 of the second glucose
This is designated as a 1,4 linkage or 1-4 bond.
Why These Numbers Matter:
Different disaccharides have different linkages:
Maltose: α-glucose + α-glucose via 1,4 bond
Sucrose: α-glucose + fructose via 1,2 bond
Lactose: β-galactose + α-glucose via 1,4 bond
In polysaccharides, the type of glycosidic bond determines structure:
1,4 bonds: Create straight chains (or helices)
1,6 bonds: Create branch points
The Condensation Reaction:
When maltose forms:
OH group on C1 of first glucose comes close to OH on C4 of second glucose
The H from one OH and the OH from the other combine to form H₂O (water)
The oxygen left behind forms the bridge: glucose-O-glucose
This is the glycosidic bond
Formation equation:
α-glucose + α-glucose → maltose + water
(C₆H₁₂O₆) + (C₆H₁₂O₆) → (C₁₂H₂₂O₁₁) + (H₂O)
Notice: 12 + 12 = 24 hydrogen atoms, but maltose only has 22, because 2H have been removed as part of water.Common Mistakes:
❌ "Beta glycosidic bond" – maltose contains α-glucose, not β-glucose
❌ "1,6 glycosidic bond" – this describes branch points in amylopectin/glycogen, not maltose
❌ Just "glycosidic" without numbers – you need to specify which carbons
❌ "Hydrogen bond" – completely wrong type of bond ❌ "Peptide bond" – that's for proteins, not carbohydrates
Examiner's Comment from Mark Scheme:
"Most candidates correctly stated that the bond was glycosidic, and many were able to achieve both marks by recognising it as a 1-4 bond. Some candidates lost the second mark by incorrectly stating that it was a 1-6 glycosidic bond."
Exam Technique Tip:
If you're ever unsure about which carbons are involved in a disaccharide bond, look for these clues:
If the molecule is described as "straight" or forms a "chain" → likely 1,4
If there's a "branch" mentioned → look for 1,6
For maltose specifically → always 1,4
Example Question 2: Hydrolysis of a Polysaccharide
Mark Scheme:
H₂O / water ✓
2 / two ✓
Alternative acceptable answers:
Award 1 mark for just H₂O/water alone
Ignore incorrect number (e.g., 3) for first mark
Model Answer:
The completed equation should read:
Maltotriose + 2H₂O → 3 glucose
Or showing the structures:
[3 glucose units joined] + 2 H₂O → 3 × [single glucose]
Detailed Explanation:
Part 1: The Substance Needed (1 mark)
The reaction requires water (H₂O). This is a hydrolysis reaction:
Hydro = water
Lysis = splitting/breaking
Hydrolysis is the opposite of condensation:
Condensation: joins monomers, removes water
Hydrolysis: breaks polymers, adds water
Part 2: The Number of Water Molecules (1 mark)
You need 2 molecules of water to break maltotriose into 3 glucose molecules.
Why 2 and not 3?
This is a crucial concept. Let's think about the bonds:
Maltotriose has three glucose units, which means:
Glucose₁—Glucose₂—Glucose₃
There are 2 glycosidic bonds (one between Glucose₁ and Glucose₂, another between Glucose₂ and Glucose₃)
Each bond requires 1 water molecule to break it
Therefore: 2 bonds = 2 water molecules
The General Rule:
For any polymer:
Number of water molecules needed = Number of monomers - 1
Examples:Disaccharide (2 monomers) → needs 1 H₂O to hydrolyse
Trisaccharide (3 monomers) → needs 2 H₂O to hydrolyse
Polysaccharide (n monomers) → needs (n-1) H₂O to hydrolyse
How Hydrolysis Works:
At each glycosidic bond:
Water molecule approaches the C-O-C bond
The O-H bond in water breaks
H⁺ attaches to one glucose oxygen
OH⁻ attaches to the other glucose carbon
The glycosidic bond breaks: C-O-C becomes C-OH and HO-C
Practical Context:
In your digestive system:
Amylase (in saliva and pancreas) breaks down starch into maltotriose and maltose
Maltase (in small intestine) then breaks these down into glucose
Each bond-breaking step is hydrolysis, requiring water
The glucose is then absorbed into your bloodstream
Common Mistakes:
❌ Writing "3 H₂O" – a common error thinking you need one water per glucose
❌ Forgetting water altogether – the bond won't break without it
❌ Writing "enzyme" instead of water – enzymes catalyse but aren't consumed
❌ Writing the number but not H₂O – you need both for full marks
Examiner's Comment from Mark Scheme:
"This question was generally well-answered. Most candidates knew that water was used for one mark and many correctly understood that two water molecules would be used in this hydrolysis reaction. Some candidates incorrectly suggested that three molecules of water were used, possibly because there were three glucose molecules."
Memory Aid:
Think of monomers as train carriages:
3 carriages are joined by 2 couplings
To separate them, you need to break 2 couplings
Each coupling break needs 1 H₂O
Total = 2 H₂O
Extension Understanding:
In polysaccharides like starch (which might have 1000+ glucose units):
Amylose with 1000 glucose units has 999 glycosidic bonds
Complete hydrolysis would require 999 water molecules
This is why digestion takes time – lots of bonds to break!
Exam Technique:
When you see questions about breaking down polymers:
Count the number of monomers (n)
Calculate bonds = n - 1
Each bond needs 1 H₂O
Always write both the substance (H₂O) and the number
Specification Point (f): The structure of starch (amylose and amylopectin), glycogen and cellulose molecules
This specification point requires detailed knowledge of the four major polysaccharides, their structural differences, and how to distinguish between them.
Example Question 1: Identifying Polysaccharide with Most 1-6 Bonds
Mark Scheme:
Correct Answer: D ✓
Model Answer: D – Glycogen
Detailed Explanation:
This question tests your understanding of how different types of glycosidic bonds create different structures in polysaccharides.
Understanding Glycosidic Bond Types:
There are two main types of glycosidic bonds in these polysaccharides:
1,4 glycosidic bonds:
Link carbon-1 of one glucose to carbon-4 of the next
Create straight chains (or helices in α-glucose polymers)
Form the "backbone" of all these polysaccharides
1,6 glycosidic bonds:
Link carbon-1 of one glucose to carbon-6 (the CH₂OH group) of another
Create branch points
Allow the chain to branch off in a new direction
Analysing Each Option:
A. Amylopectin (INCORRECT)
Structure: Branched, but with relatively few branches
Branching frequency: Approximately every 20-25 glucose units
Percentage of 1,6 bonds: About 4-5%
Has 1,6 bonds, but not the highest proportion
B. Amylose (INCORRECT)
Structure: Completely unbranched helical chain
Contains ONLY α-1,4 glycosidic bonds
Percentage of 1,6 bonds: 0%
Forms a coiled helix due to the angle of 1,4 bonds in α-glucose
C. Cellulose (INCORRECT)
Structure: Completely unbranched straight chains
Contains ONLY β-1,4 glycosidic bonds
Percentage of 1,6 bonds: 0%
Forms straight chains because alternate glucose units are rotated 180°
D. Glycogen (CORRECT) ✓
Structure: Highly branched, with many branch points
Branching frequency: Approximately every 8-12 glucose units
Percentage of 1,6 bonds: About 8-10%
Has the highest proportion of 1,6 bonds of all options
Why Glycogen Has More 1-6 Bonds:
Glycogen is essentially a "super-branched" version of amylopectin:
The Biological Reason:
Why does glycogen have so many more branch points?
Rapid energy release: Animals need to release glucose quickly for sudden energy demands (running from predators, chasing prey, exercise)
More enzyme access: Each branch point creates a "free end" where enzymes can work. More branches = more free ends = faster breakdown
Compact storage: More branching creates a more spherical, compact molecule – important for animals that move around
Higher metabolic rate: Animals generally have higher metabolic rates than plants, so need faster access to stored glucose
Common Mistakes:
❌ Choosing A (Amylopectin) – the most common wrong answer. Students know it's branched but don't realise glycogen is MORE branched
❌ Not understanding "proportion" – the question asks for highest proportion, not just "which one has 1-6 bonds"
❌ Confusing structure with function – knowing glycogen stores energy doesn't help if you don't know its structural details
Examiner's Comment from Mark Scheme:
"Around 4 out of 5 candidates selected the correct response, option D, showing good understanding of glycosidic bonds and polysaccharides. Option A was the most common incorrect response."
Key Learning Points:
0% 1,6 bonds: Amylose and cellulose (unbranched)
~4% 1,6 bonds: Amylopectin (some branches)
~10% 1,6 bonds: Glycogen (highly branched) ← HIGHEST
Memory Aid:
Think: "Glycogen = Greatly branched = Greatest proportion of 1-6 bonds"
Or remember: Animals are more active → need faster energy → more branches → most 1-6 bonds
Example Question 2: Comparing Amylose and Cellulose Structures
Mark Scheme:
Award 1 mark for each correct row irrespective of which box contains the information.
Acceptable answers (any three from):
(contains) α / alpha / A / a (glucose) (contains) β / beta / B / b (glucose)
α / alpha / A / a 1-4 glycosidic bonds β / beta / B / b 1-4 glycosidic bonds
all monomers / AW, same orientation alternate monomers at 180° / AW, to each other
granular / not fibrous fibrous / not granular
H bonds within molecule / no (H) bonds between molecules (H) bonds between adjacent molecules
Guidance: ✓ Accept "every second one is flipped" ✓ Accept fibres / microfibrils / fibrils / macrofibrils ✗ Do NOT credit myofibrils (that's muscle, not cellulose!) ✓ Accept grains for granular ✓ Accept '(cross)links' for 'bonds'
Model Answer:
Amylose Cellulose coiled no coiling contains α-glucose contains β-glucose α-1,4 glycosidic bonds β-1,4 glycosidic bonds all glucose units same orientation alternate glucose units rotated 180°
Or alternatively:
Amylose Cellulose coiled no coiling granular structure fibrous structure no H bonds between chains H bonds between adjacent chains all glucose same way up every other glucose flipped
Detailed Explanation:
Let's explore each structural difference and why it matters:
Difference 1: Type of Glucose Monomer
Amylose: Made from α-glucose
OH group on carbon-1 is below the ring
When joined, all glucose units face the same direction
This creates the possibility of coiling
Cellulose: Made from β-glucose
OH group on carbon-1 is above the ring
Each successive glucose must be rotated 180° to allow bonding
This creates straight chains
Why it matters: The single structural difference in the monomer (OH position on C1) determines whether the polymer coils or forms straight chains.
Difference 2: Type of Glycosidic Bond
Amylose: Contains α-1,4 glycosidic bonds
Links α-glucose monomers
Can be broken by human digestive enzymes (amylase, maltase)
Digestible!
Cellulose: Contains β-1,4 glycosidic bonds
Links β-glucose monomers
Cannot be broken by human digestive enzymes
Indigestible (we lack cellulase enzyme)
Why it matters: This explains why we can digest starch (bread, pasta, potatoes) but not cellulose (grass, wood, paper), even though both are glucose polymers!
Difference 3: Orientation of Monomers
Amylose: All glucose units in same orientation
Every glucose faces the same direction
The CH₂OH groups all project to one side
Allows the molecule to coil into a helix
Cellulose: Alternate glucose units rotated 180°
Every other glucose is flipped
The CH₂OH groups alternate sides
Forces the molecule to remain straight
Why it matters: The alternating orientation is WHY β-glucose forms straight chains – it's structurally impossible for cellulose to coil when alternate monomers face opposite directions.
Difference 4: Overall Shape
Amylose: Coiled / helical
Forms a spiral/helix (like a spring or telephone cord)
The helix is stabilized by hydrogen bonds within the same molecule
Typically 6 glucose units per turn of the helix
Creates a compact structure
Cellulose: Straight chains / no coiling
Remains completely linear
Multiple chains lie parallel to each other
No bending or twisting
Forms microfibrils (bundles of ~60-70 chains)
Why it matters: The straight chains of cellulose can pack tightly together and form extensive hydrogen bonds between chains, creating incredible tensile strength.
Difference 5: Hydrogen Bonding Pattern
Amylose: H bonds within the same molecule
Hydrogen bonds form within the coiled structure
These stabilize the helix
No (or minimal) bonding between separate amylose molecules
Molecules remain separate and granular
Cellulose: H bonds between adjacent molecules
Extensive hydrogen bonding between parallel chains
OH groups on one chain hydrogen bond to OH groups on neighbouring chains
Creates cross-links between chains
Bundles chains together into microfibrils
Why it matters: The inter-chain hydrogen bonding in cellulose is what gives it exceptional strength – comparable to steel! Plant cell walls can withstand enormous pressures because of this.
Difference 6: Physical Form
Amylose: Granular
Forms discrete grains or granules
Stored in starch grains in chloroplasts (plants)
Individual molecules don't form fibres
Appears as white powder when extracted
Cellulose: Fibrous
Forms long fibres (microfibrils → macrofibrils)
These fibres are embedded in the plant cell wall
Gives cell walls their strength and structure
Visible as stringy material (think celery strings)
Why it matters: The fibrous nature is essential for cellulose's structural role, whilst the granular nature suits amylose's storage role.
Common Mistakes:
❌ Writing about glycogen or amylopectin – the question specifically asks about amylose
❌ Using "myofibrils" – that's muscle tissue, not plant cells!
❌ Not comparing like with like – saying "amylose is coiled" and "cellulose contains β-glucose" in the same row doesn't work
❌ Describing function instead of structure – "amylose stores energy" vs "cellulose provides strength" won't gain marks
❌ Adding a 4th or 5th row – only the first 3 rows after the given row are marked
Examiner's Comment from Mark Scheme:
"This question was not answered well. Most candidates gained 1 or 2 marks, usually for identifying α- and β-glucose as subunits, the fibrous nature of cellulose or the arrangement of hydrogen bonding. Few got full marks. A significant minority used terms associated with protein structure and gained no credit. Similarly, many candidates gave differences relating to function rather than structure and gained no credit."
Exam Technique:
When answering comparison tables:
Read what's already provided – use it as a clue for the level of detail needed
Keep comparisons in the same row – left box should relate to right box
Use "vs" thinking – α vs β, coiled vs straight, within vs between
Stick to structure, not function – unless the question explicitly asks for function
Check you've filled enough rows – but not too many (only first 3 marked)
Specification Point (g): How the structures and properties of glucose, starch, glycogen and cellulose molecules relate to their functions in living organisms
This is arguably the most important specification point – linking molecular structure to biological function. This type of question appears repeatedly and often carries high mark allocations.
Example Question 1: Properties and Functions of Glucose, Starch and Glycogen
Mark Scheme:
Glucose:
soluble / polar ✓
has chemical energy in its bonds OR is a respiratory substrate / source of energy ✓
Starch / Glycogen:
insoluble and compact OR large(r) SA ✓
used for (energy / glucose) storage / allows quick release (of stored energy / glucose) ✓
idea that glycogen is broken down faster than starch due to higher SA / many branch ends ✓
Guidance: ✗ Ignore descriptions of structure (e.g., 'glycogen is branched') ✗ Ignore misspelling of 'glycogen' throughout ✓ Allow "releases energy/ATP" ✗ Ignore "starch/glycogen can be stored" ✗ Ignore "broken down more easily" (needs to be about speed/rate)
Maximum 4 marks total
Model Answer:
Glucose properties and functions: "Glucose is soluble in water because it's a polar molecule with many OH groups that can form hydrogen bonds with water. This allows glucose to be transported in solution in blood plasma (animals) or phloem sap (plants).
Glucose contains chemical energy stored in its C-H and C-C bonds. When these bonds are broken during respiration, the energy is used to synthesize ATP. Glucose is therefore the primary respiratory substrate that provides energy for cellular processes."
Starch and glycogen properties and functions: "Both starch and glycogen are insoluble in water, so they have no osmotic effect on cells. This means they can be stored in large quantities without affecting the cell's water potential or causing water to move into the cell by osmosis, which would cause the cell to swell and potentially burst.
Both molecules are compact (especially glycogen with its highly branched structure), taking up relatively little space whilst storing large amounts of glucose. This allows cells to store substantial energy reserves without requiring excessive volume.
Both serve as energy storage molecules that can be hydrolysed to release glucose when energy is needed. However, glycogen is broken down more rapidly than starch because it is more highly branched. The greater number of branch points (1-6 glycosidic bonds) creates many free ends where enzymes can simultaneously attach and break off glucose units. This rapid mobilization is essential for animals, which have higher metabolic rates and may need sudden bursts of energy."
Detailed Explanation:
Let's break down how to construct perfect structure-function links:
GLUCOSE – Property 1: Solubility
Structure → Property → Function chain:
Structure:
Small molecule (C₆H₁₂O₆)
Contains 5 hydroxyl (OH) groups
Polar molecule
Property:
Soluble in water (hydrophilic)
Can dissolve to high concentrations
Function:
Can be transported in aqueous solutions:
In blood plasma (animals)
In phloem sap (plants)
Through cytoplasm
Can be delivered to all cells that need energy
Crosses cell membranes via specific transport proteins
Why this matters: If glucose weren't soluble, it couldn't be distributed around organisms. The blood glucose concentration of ~5 mM provides a constant supply to respiring cells.
GLUCOSE – Property 2: Energy Content
Structure → Property → Function chain:
Structure:
Contains multiple C-H bonds
Contains C-C bonds
Contains C-O bonds
Ring structure can be opened and broken down
Property:
Energy-rich molecule
Contains ~2880 kJ of energy per mole
Relatively unstable (can be oxidized)
Function:
Primary respiratory substrate
Broken down in glycolysis → Krebs cycle → electron transport chain
Produces ATP for cellular processes
Provides energy for:
Active transport
Synthesis of molecules
Movement
Cell division
Maintaining body temperature
Why this matters: Glucose is the universal cellular fuel. Nearly all organisms can respire glucose to release energy.
STARCH/GLYCOGEN – Property 1: Insolubility
Structure → Property → Function chain:
Structure:
Very large molecules (polymers of thousands of glucose units)
Compact, coiled/branched shape
Few free OH groups on the exterior
Property:
Insoluble in water
Does not dissolve
Metabolically inactive
Function:
Can be stored without osmotic effects:
Doesn't affect water potential (Ψ)
No water drawn into cells by osmosis
Cells don't swell or burst
Doesn't interfere with cell metabolism
Can store large quantities safely
Why this matters: Imagine if cells stored glucose instead:
If a liver cell stored the same amount of energy as glucose instead of glycogen, the osmotic effect would draw in so much water the cell would burst!
Glucose concentration of equivalent energy would be ~400 mM (compared to blood's 5 mM), creating huge osmotic gradient
STARCH/GLYCOGEN – Property 2: Compactness
Structure → Property → Function chain:
Structure:
Coiled (amylose) or branched (amylopectin/glycogen) structure
Folds into dense, compact shape
Glycogen especially: highly branched → spherical shape
Property:
Very compact
High energy density
Small volume:energy ratio
Function:
Can store large amounts of energy in small space:
Liver cells packed with glycogen granules
Starch grains in chloroplasts
Doesn't take up excessive cell volume
Leaves space for other organelles and processes
Particularly important in animals that need to move
Why this matters:
Humans store about 400g of glycogen (liver + muscles)
This provides about 1600 kcal of energy
If stored as glucose, it would require massive amounts of space and create devastating osmotic problems
STARCH/GLYCOGEN – Property 3: Storage and Release
Structure → Property → Function chain:
Structure:
Polymer of α-glucose units
Connected by α-glycosidic bonds
Can be hydrolysed by enzymes we possess
Property:
Can be easily broken down by enzymes
Hydrolysis releases glucose monomers
Reversible synthesis/breakdown
Function:
Stores glucose for later use
Can be mobilized when energy needed:
During exercise (animals)
At night when no photosynthesis (plants)
During fasting/starvation
Glucose released enters cellular respiration
Why this matters: This provides a buffer between energy supply and demand – organisms don't need constant food intake.
GLYCOGEN vs STARCH – The Critical Comparison
Structure → Property → Function chain:
Glycogen Structure:
Highly branched (branch every ~10 glucose)
More 1-6 glycosidic bonds (~10%)
More compact and spherical
Starch (Amylopectin) Structure:
Less branched (branch every ~25 glucose)
Fewer 1-6 glycosidic bonds (~4%)
Less compact
Property:
Glycogen has many more free ends
Greater surface area for enzyme attachment
More sites for simultaneous hydrolysis
Function:
Glycogen can be broken down more rapidly
Releases glucose faster when needed
Suits animals with:
Higher metabolic rates
Need for sudden energy bursts
Active lifestyles
Why this matters:
A cheetah chasing prey needs instant glucose release from muscles
A plant growing slowly over months doesn't need such rapid mobilization
The structural difference (branching frequency) directly determines functional difference (release rate)
Examiner's Comment from Mark Scheme:
"An excellent discriminator with only the most able candidates achieving the full 4 marks in a well-organised and concise response. Almost all candidates had some knowledge to share even if it was often confused and organised poorly. Less able candidates described the general structure of the carbohydrates while a few included the structure of cellulose. The most frequently given marks were glucose being soluble, glucose being used in respiration and starch or glycogen being used for storage. Some common mistakes included: easy release of glucose from the polysaccharides rather than rapid release, or not comparing the potential rate of release in glycogen to that in starch."
Common Mistakes:
❌ Describing structure without linking to function: "Glycogen is branched" (so what? why does that matter?) ✓ Correct approach: "Glycogen is highly branched, which creates many free ends where enzymes can work, allowing rapid glucose release"
❌ Vague function statements: "Glucose provides energy" ✓ Better: "Glucose is a respiratory substrate that releases energy when oxidized during aerobic respiration"
❌ Missing the comparison: Discussing glycogen and starch separately without comparing their rate of breakdown ✓ Correct: "Glycogen is broken down faster than starch because it has more branch points"
❌ Confusing "easy" with "fast": "Glycogen is easily broken down" ✓ Correct: "Glycogen is broken down rapidly/quickly"
❌ Including irrelevant structures: Discussing cellulose in an answer about energy storage
Top Exam Technique Tips:
Use the Structure → Property → Function framework
Don't just say "glucose is soluble" – explain that small size and OH groups make it polar, so it dissolves in water, so it can be transported in blood
Make explicit comparisons when asked
Use comparative language: "more than", "faster than", "unlike"
"Glycogen has MORE branches than starch, creating MORE free ends, allowing FASTER breakdown"
Link to real biology
"This is important because animals need rapid energy release for movement/exercise"
Shows you understand why these properties matter
Organize your answer clearly
Write about glucose first
Then starch and glycogen together (their similarities)
Then glycogen vs starch (their differences)
Use precise terminology
"Osmotic effect" not just "affects the cell"
"Respiratory substrate" not just "gives energy"
"Hydrolysed" not just "broken down"
Example Question 2: Why Store Glycogen Instead of Glucose?
Mark Scheme:
Any three from:
Glycogen is:
insoluble, so has no effect on water potential / Ψ (of cell) ✓
metabolically inactive ✓
compact / lots can be stored in a small space ✓
able to store large amounts / lots of energy ✓
(highly branched so) has lots of ends for adding / removing glucose (when needed) OR can be broken down fast / quickly / rapidly to release glucose ✓
Guidance: ✓ Accept ORA (or reverse argument) for glucose for points 1, 2, 3 & 4 only ✓ For point 1: Accept "insoluble so has no osmotic effect (on cell)" ✗ For point 5: Ignore references to surface area ✗ For point 5: Ignore "energy release" in this context
Note: "Compact so can store large amounts of energy" = 2 marks (points 3 & 4)
Model Answer:
"Mammals store glycogen rather than glucose because:
Glycogen is insoluble, whereas glucose is soluble. This means glycogen has no osmotic effect on cells. If glucose were stored at high concentrations, it would lower the cell's water potential dramatically, causing water to move into the cell by osmosis, potentially causing the cell to swell and lyse (burst). Glycogen storage avoids this problem entirely.
Glycogen is very compact due to its highly branched structure, allowing large amounts of glucose to be stored in a small volume. A liver cell can store far more energy as glycogen granules than it could as free glucose without taking up excessive space needed for other cellular structures and processes.
Glycogen's highly branched structure creates many free ends (branch points) where enzymes can simultaneously attach. This allows glucose to be rapidly released when energy is needed – essential for mammals with high metabolic rates or during exercise when energy demands suddenly increase."
Detailed Explanation:
This question is asking you to compare storing as glycogen versus storing as glucose. Let's explore each advantage in detail:
Advantage 1: No Osmotic Problems (MOST IMPORTANT)
The Problem with Storing Glucose:
If a liver cell tried to store the same amount of energy as free glucose molecules instead of glycogen:
A typical liver cell stores about 8% of its mass as glycogen
This represents thousands of glucose molecules per glycogen molecule
If stored as free glucose, the concentration would be approximately 400 mM
Compare this to normal blood glucose: 5 mM
The osmotic catastrophe:
Water potential (Ψ) = Pressure potential (Ψp) + Solute potential (Ψs)
High glucose concentration means:
Very negative solute potential inside the cell
Very negative water potential inside the cell
Water potential outside cell is much higher (less negative)
Water moves into the cell by osmosis down the Ψ gradient
Cell swells and potentially bursts (lysis)
The glycogen solution:
Glycogen molecules are huge (molecular mass: 1-10 million Da)
One glycogen molecule might contain 60,000 glucose units
But it only contributes 1 particle to osmotic concentration
Effectively reduces osmotic concentration by 60,000-fold
No significant osmotic effect on the cell
Analogy: It's like the difference between having:
60,000 individual pennies scattered in a room (very cluttered, affects space)
ONE £600 note (same value, negligible space)
Advantage 2: Compact Storage / Space Efficiency
Physical Compactness:
Glycogen structure:
Highly branched (every 8-12 glucose units)
Branches branch further (up to 12 tiers of branching)
Forms a roughly spherical, compact granule
Dense, tightly packed structure
Glucose storage:
Individual small molecules
Would be dispersed throughout cytoplasm
Cannot pack efficiently
Would fill the cell
Quantitative Comparison:
In a liver cell:
As glycogen: ~100-400 glycogen granules, each about 10-40 nm diameter
As glucose: Would require the same number of molecules but dispersed, occupying far more cytoplasmic space
Energy Density:
Glycogen: High energy density – lots of energy in small volume
Glucose: Low energy density – same energy needs huge volume
Why this matters for mammals:
Mammals need to:
Move – excess weight/volume is disadvantageous
Maintain other cell functions – need space for organelles
Store substantial reserves – might not eat for hours
A human stores about 400g of glycogen (liver + muscles):
This provides ~1600 kcal of readily available energy
Enough for about 90 minutes of running
If stored as glucose, would require impossible amounts of space
Advantage 3: Rapid Mobilization
Structural Basis:
Glycogen's branching pattern:
Branch every 8-12 glucose units
Creates many free ends (non-reducing ends)
Each branch point is a 1-6 glycosidic bond
Enzyme Action:
Glycogen phosphorylase removes glucose units from free ends
More free ends = more enzyme binding sites
Multiple enzymes can work simultaneously
Result: Rapid release of many glucose molecules at once
Quantitative Effect:
Imagine a glycogen molecule with 10,000 glucose units:
If unbranched (like amylose): Only 2 free ends (one at each end of the chain)
If highly branched (like glycogen): Potentially hundreds of free ends
Rate of glucose release:
Unbranched: Limited by having only 2 sites for enzyme action
Highly branched: Dramatically faster due to hundreds of simultaneous sites
Why this matters for mammals:
Mammals frequently need sudden energy bursts:
Exercise: Muscle contraction requires immediate ATP
Glycogen in muscles broken down rapidly
Releases glucose for respiration
Provides ATP within seconds
Fight or flight: Stress response needs quick energy
Adrenaline triggers glycogen breakdown
Liver releases glucose into blood
Raises blood glucose rapidly
High metabolic rate: Mammals are endotherms
Maintain constant body temperature
Requires continuous energy supply
Need ability to quickly access reserves
Contrast with plants:
Plants don't move
Lower metabolic rate
Less urgent energy demands
Can use less-branched starch (amylopectin)
Additional Advantage: Metabolically Inactive
What this means:
Glycogen doesn't participate in other metabolic reactions
It's chemically inert until deliberately broken down
Won't interfere with cellular processes
Stable storage form
Why this matters:
Glucose is reactive – enters many metabolic pathways:
Glycolysis (immediate breakdown)
Pentose phosphate pathway
Protein glycosylation
Production of other sugars
Storing as glucose would make it immediately available for metabolism
Can't build up reserves if it's constantly being used
Glycogen provides a reservoir that's only tapped when needed
Common Mistakes:
❌ "Glycogen stores energy" – too vague, doesn't explain WHY it's better than glucose ✓ Better: "Glycogen can store large amounts of energy in a small space"
❌ "Glycogen is easily broken down" – the word "easily" doesn't credit ✓ Better: "Glycogen can be broken down rapidly/quickly"
❌ "Glycogen has no osmotic potential" – incorrect terminology ✓ Better: "Glycogen has no effect on water potential" or "no osmotic effect"
❌ "Glycogen has more surface area" – mark scheme says to ignore this ✓ Better: "Glycogen has many free ends where enzymes can work"
❌ Missing the comparison – only describing glycogen, not explaining why it's better than glucose ✓ Better: Explicitly compare: "Unlike glucose, glycogen..."
Examiner's Comment from Mark Scheme:
"Candidates understood that glycogen is more compact than glucose, but didn't usually go on to explain that it stores large amounts of energy. Many commented that glycogen is insoluble, but didn't explain that it can be stored without any water potential implications for cells. A large number of candidates substituted 'energy' for 'glucose' when describing how the structure of glycogen allows a rapid release of glucose. There was a tendency to describe removal of glucose as 'easy' rather than 'fast'."
Perfect Answer Structure:
A full-mark answer would be:
"Glycogen is insoluble, so has no osmotic effect on liver cells – unlike glucose which would draw water into cells and potentially cause them to burst.
Glycogen is very compact, allowing large amounts of energy to be stored in a small space – this is essential for mammals that need to move.
Glycogen is highly branched with many free ends, allowing rapid release of glucose when energy is needed – important for mammals' high metabolic rates and sudden energy demands."
This hits 5 marking points but you only need 3 for full marks!
Memory Technique:
Remember "I-C-R":
Insoluble → no osmotic problems
Compact → space-efficient storage
Rapid → fast mobilization when needed
Summary Table: Specification Point (g)
Final Checklist for Specification 2.1.2 (d-g)
Before your exam, ensure you can:
For point (d):
✅ Draw α-glucose and β-glucose accurately, showing OH position on C1
✅ Explain that glucose is a hexose (6C) and ribose is a pentose (5C)
✅ Complete partial ring structures for both glucose and ribose
✅ Count carbon atoms in ring structures correctly
For point (e):
✅ Name glycosidic bonds precisely (α-1,4, α-1,6, β-1,4)
✅ Describe condensation reactions (remove H₂O, form glycosidic bond)
✅ Describe hydrolysis reactions (add H₂O, break glycosidic bond)
✅ Calculate water molecules needed: n monomers need (n-1) H₂O to break apart
✅ Name the disaccharides: maltose, sucrose, lactose
For point (f):
✅ Compare amylose, amylopectin, glycogen, and cellulose structures
✅ State which contain α-glucose (amylose, amylopectin, glycogen) vs β-glucose (cellulose)
✅ State which are branched (amylopectin, glycogen) vs unbranched (amylose, cellulose)
✅ Explain that 1,6 bonds create branch points
✅ Explain that glycogen has more branches than amylopectin
For point (g):
✅ Explain why glucose is soluble and how this aids transport
✅ Explain why glucose is a good respiratory substrate
✅ Explain why starch/glycogen are insoluble (osmotic advantages)
✅ Explain why starch/glycogen are compact (space efficiency)
✅ Explain why glycogen releases glucose faster than starch (more branch points)
✅ Explain why cellulose is strong (H bonds between chains)
✅ Link structure to function using "this allows/enables/means that..."
Recommended Revision Activities
Create flashcards with structures on one side, properties and functions on the other
Draw and redraw glucose structures until you can do them perfectly from memory
Make comparison tables like the one above – creating them yourself aids memory
Practice past paper questions using the examples in this guide, then check the mark schemes
Teach someone else – if you can explain it clearly to another person, you truly understand it
Use the "Structure → Property → Function" framework for every molecule
Create mind maps linking all four specification points together
Final Words
Carbohydrates are fundamental to life and form a significant portion of your A Level Biology course. The key to success is understanding why structures lead to particular properties, and why those properties suit specific functions.
Don't just memorize facts – understand the logic:
Small + soluble = good for transport (glucose)
Large + insoluble = good for storage (starch/glycogen)
Many branches = fast release (glycogen)
Straight chains with H bonds = strong (cellulose)
With the detailed examples and mark schemes in this guide, you now have everything you need to achieve top marks on specification points 2.1.2 (d-g).
Good luck with your studies! 🧬
Remember: Practice doesn't make perfect – practice with detailed feedback makes perfect. Use these mark schemes to understand not just what to write, but WHY those answers gain marks.